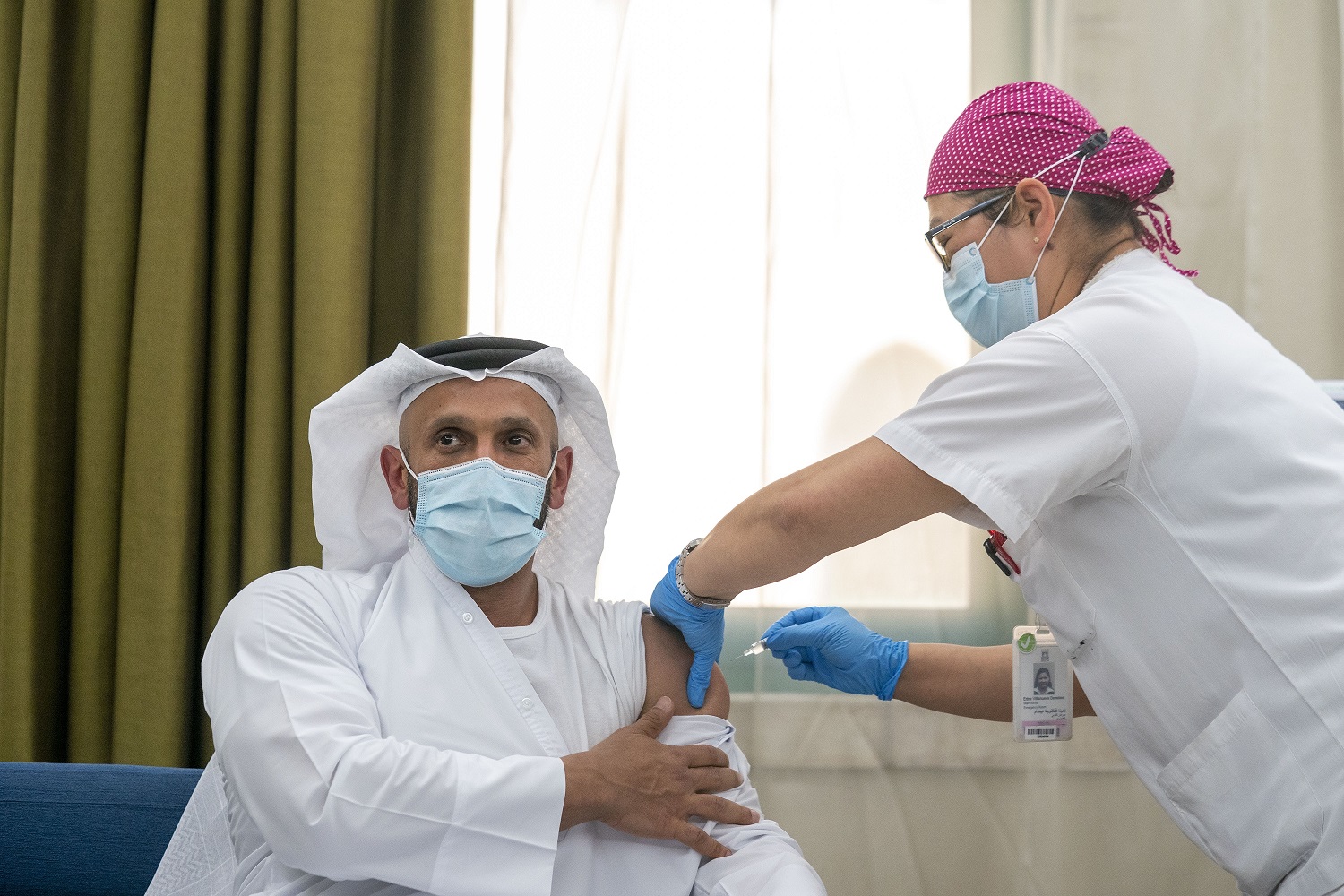
The first WHO enlisted global clinical Phase III trial of Sinopharm CNBG’s inactivated vaccine to combat COVID-19 has started in Abu Dhabi in the United Arab Emirates, and has been inspired by the UAE Leadership’s vision and commitment to overcome the pandemic through a global collaborative effort.
H.E. Sheikh Abdullah bin Mohammed Al Hamed, Chairman of the Department of Health, Abu Dhabi, was the first individual in the world to commence the trial of a Phase III inactivated vaccine for COVID-19. The Department’s Acting Undersecretary, Dr. Jamal Al Kaabi was the second volunteer to trial the vaccine showcasing the commitment of the UAE Government and the Health Authorities to find a cure for humanity’s biggest challenge of the 21st century.
The world’s first Phase III trial is the result of a cooperation partnership between Abu Dhabi based G42 Healthcare, currently at the forefront of the battle against COVID-19 in the UAE,and Sinopharm CNBG, the world’s sixth largest vaccine manufacturer, ranked 169th on the Fortune Global 500 list of 2018.
The trials are being operated by health practitioners from Abu Dhabi Health Services (SEHA) who are providing facilities at five of their clinics in Abu Dhabi and Al Ain in addition to a mobile clinic to ensure the trials are readily accessible to volunteers participating in the programme.
Over the past few months, G42 Healthcare meanwhile has established a massive throughput laboratory to speed up the detection of the disease; manufactured essential PPE; conductedresearch into new vaccines and drug therapies, and used its advanced AI capabilities to map and predict trends in the outbreak, virus mutationsand help combat the disease.
The UAE was the preferred choice for the cooperation partnership to conduct the Phase III trials for the inactive vaccine as the nation is home to over 200 nationalities, allowing for robust research across multiple ethnicities and increasing its feasibility for global application on the success of the trials.
The UAE Health Authorities have recently issued a permit for up to 15,000 volunteers to take part in the trials. G42 Healthcare and SEHA areworking towards achieving a minimum of 5,000 participantsin the first stage of the programme to ensure the robustness of the results.
Today’s clinical trial commencement is the start of a series of national initiatives to both foster population health and to enhance the UAE’s medical research and development capabilities, including the local capacity to manufacture the vaccine.
Trial formally began inthe presence of the Chinese Ambassador to the UAE, H.E Ni Jian; senior health department officials and G42 Healthcare and Sinopharm CNBG representatives.The first group of volunteers including UAE nationals and expatriates received the vaccine at Sheikh Khalifa Medical City today.
The clinical trials are being conducted under the strict guidance and supervision of the Department of Health Abu Dhabi and SEHA – the Abu Dhabi Health Services Company. The trials follow all international guidelines stipulated by the World Health Organization (WHO) and the United States Food & Drug Authority (USFDA).
The study, if successful, will be approved and accredited by the Ethics Committee for Scientific Research in the Emirate of Abu Dhabi.
Commenting on the start of the programme, UAE Principle Investigator Sheikh Khalifa Medical City CMO and Chairpersonof the National COVID-19 Clinical Management Committee Dr. Nawal Ahmed Alkaabi said: “Our participation in this trial enables us to make a major contribution in the global fight to combat the COVID-19 pandemic. It is a matter of national pride that we are able to help facilitate the trial process that could have a worldwide impact and help people around the world to benefit from research and – if successful – the manufacture of a vaccine to fight back against this disease.”
The phase III clinical trial follows the success of the phase I and phase II trials conducted by Sinopharm in China, which resulted in 100% of the volunteers generating antibodies after two doses in 28 days. The phase III trials will be open to individual volunteers aged between 18 and 60 living in Abu Dhabi and Al Ain and will last for 42 days.
A key factor forCOVID-19 vaccine is the urgency around global implementation. Computing power, data processing and diagnostic analysis are G42 Healthcare’s global competitive advantage to support the successful delivery of the world’s first phase III trials of inactivated vaccine.
G42 Healthcare CEO Ashish Koshy said: “We are enormously proud that Sinopharm CNBG has partnered G42 Healthcare in this groundbreaking phase III clinical trial in the UAE. Using our AI solutions, super-computer, advanced diagnostics solutions for COVID-19, G42 Healthcare is uniquely postured to conduct these trials.G42 Healthcare will be responsible for running clinical operations for this trial. We will be leveraging our group’s technical and our own business capabilities to:compute, correlate and provide fast and synthesized insights by deploying multiple AI models on the data generated during the trials to accelerate the much awaiting results. G42 Healthcarewill be mobilizing the logistical management of the trials taking in learnings from its proven capabilities in CRO management, clinical sites initiations and other E2E programme management activities.”
Mr. Jingjin Zhu, President, Biological products, Sinopharm CNBG added: “The United Arab Emirates is a nation of innovation and tolerance, that is home to individuals from every part of the world and ethnic background. We will work closely with our partner to complete this clinical trials successfully, and make this vaccine available to the people in need worldwide. With the full support of local authorities, cutting-edge technologies provided by our partner G42 Healthcare, and high-quality services and supports from the medical and clinical entities, we will jointly contribute to the battle against COVID-19 worldwide.
Now that the trials have officially commenced, G42 Healthcare and the UAE Health Authorities will shortly launch a public awareness campaign to encourage UAE residents to participate in thiscritical to humanity clinical trial programme.